How DMCC differs
DMCC is an advanced multi-solvent liquid–liquid chromatographic platform designed for high-resolution purification of a wide variety of chemical compounds. By continuously passing the mobile phase through a series of connected tubes filled with stationary phase, compounds are separated based on their differential solubility between immiscible solvents.
Core Principles & Components
Differential Distribution
Compounds are separated according to their solubility differences between mobile and stationary phases.
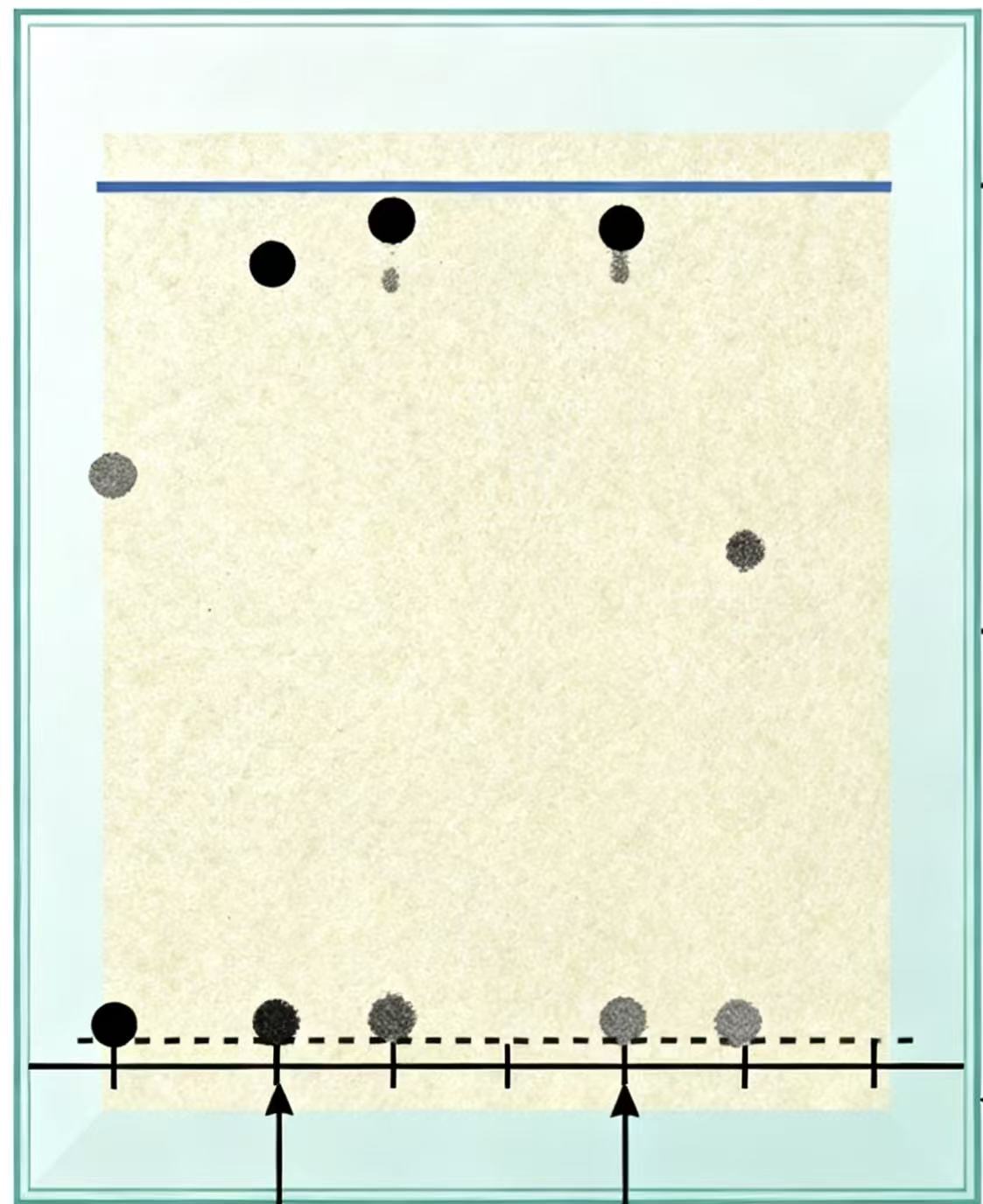
Continuous Flow with Nanoparticle Dispersion
The mobile phase is dispersed into nanoparticles as it enters the column, enhancing contact with the stationary phase and improving separation efficiency.
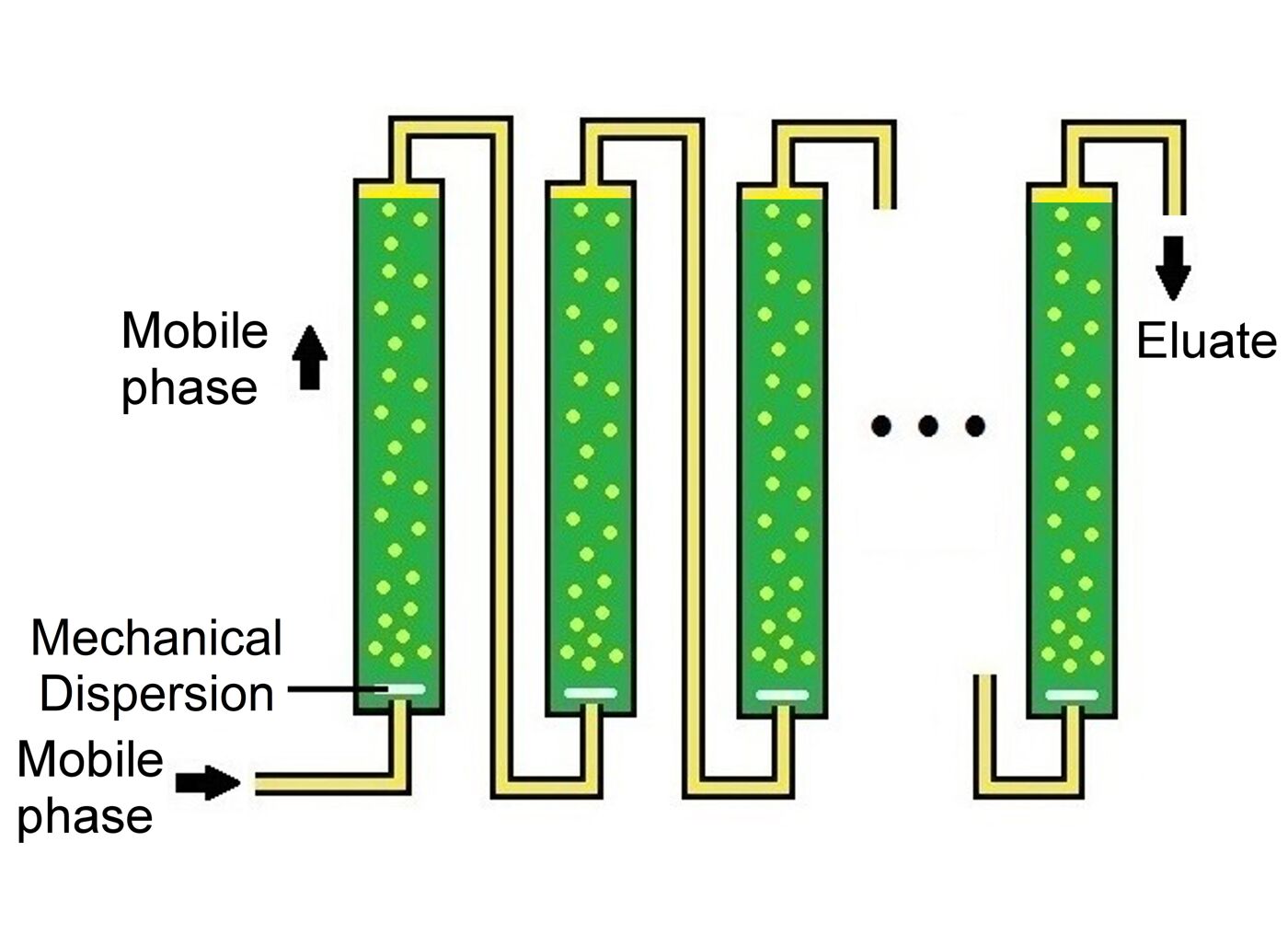
Series Tubular Design
Multiple tubes are connected in series, allowing continuous purification at large scale without solid resins or centrifugation.
Fraction Collection
High-precision collectors with integrated analytics to minimize cross-contamination and solvent usage.

DMCC Advantages
Cost Effective
Save over 50% in equipment, solvent, maintenance, and waste disposal costs.
Highly Customizable
Easily switch solvent systems for different types of molecules.
Eco-Friendly
Stationary phase is easily evaporated, solvents are reusable, and there is no solid waste pollution.
Highly Scalable
Production can be easily scaled up to kilogram-level purification by adjusting parameters.
Safe Operation
Operates under low pressure without centrifugation, ensuring safety and reliability.
High Resolution
Capable of separating structurally similar compounds such as enantiomers.
Technology in Action — Interactive Diagram

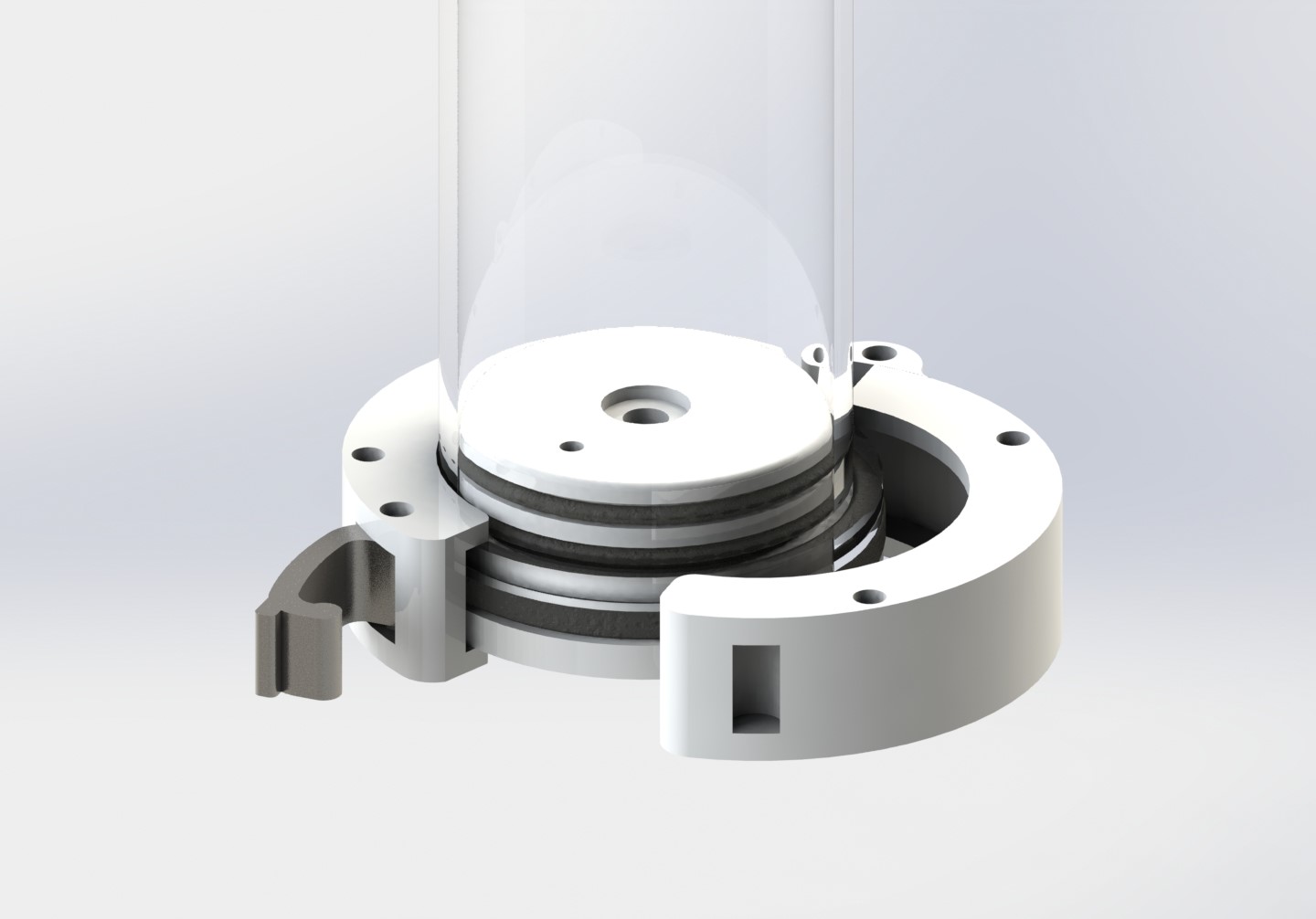
Tube connection using flangeless fittings for leakage prevention
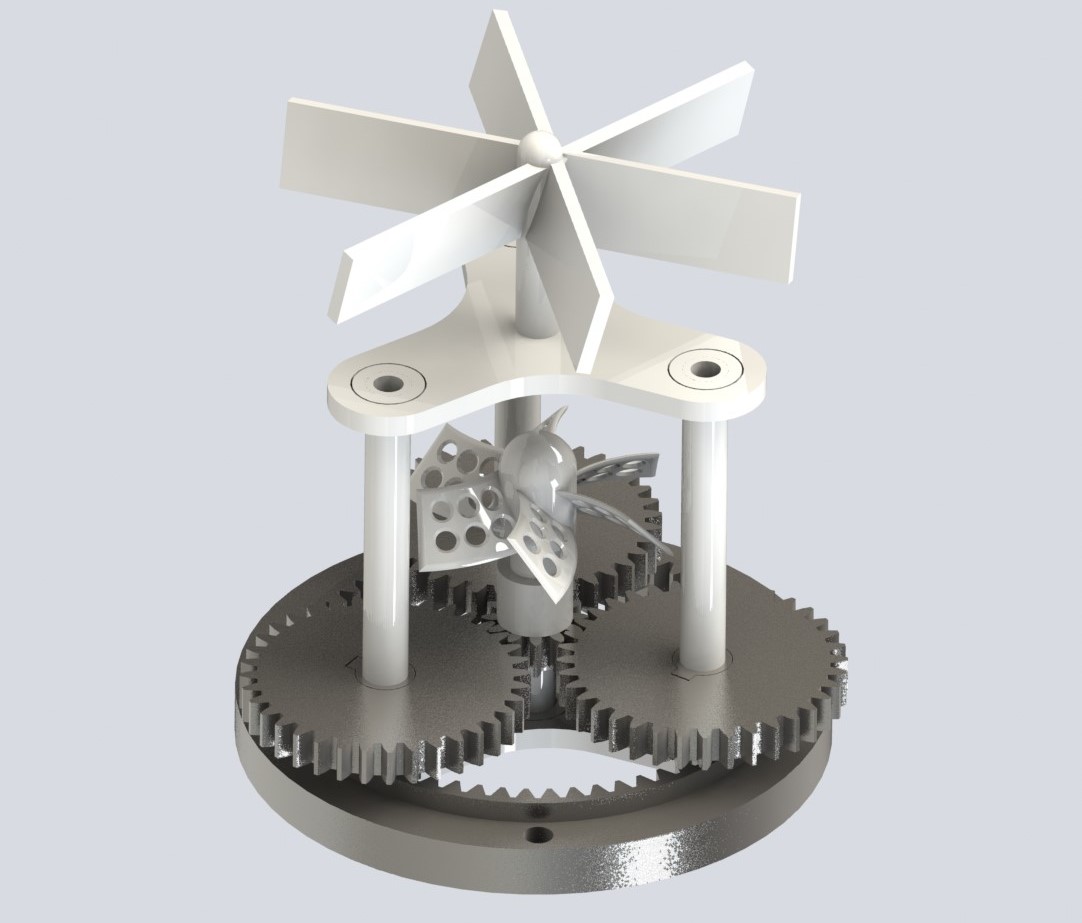
Slide-aligned coupling design for motor–fan transmission
One-click assembly between column and bottom part
Hover hotspots
Hover on the diagram to see DMCC modules
Applications & Case Studies
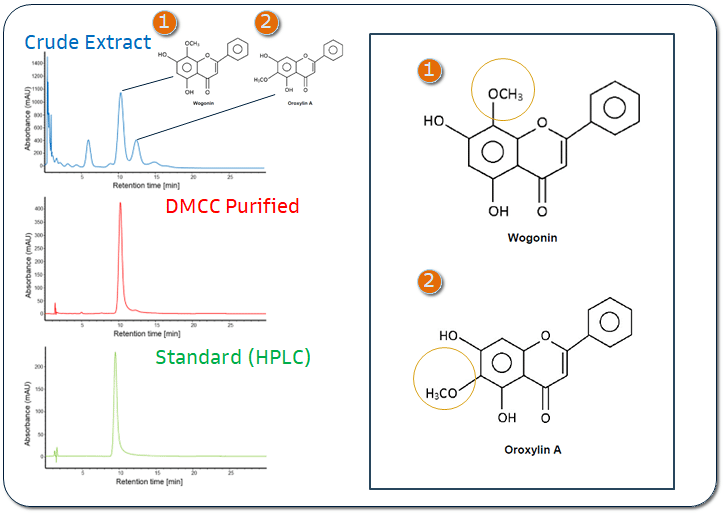
Cost-Efficient Scaled Production
Achieved industrial-scale purification of Wogonin with approximately 80% lower production cost compared with conventional market processes.

High-Purity Pharmaceutical Grade
Successfully purified Baicalein to pharmaceutical-grade purity with HPLC-verified results reaching 98%.
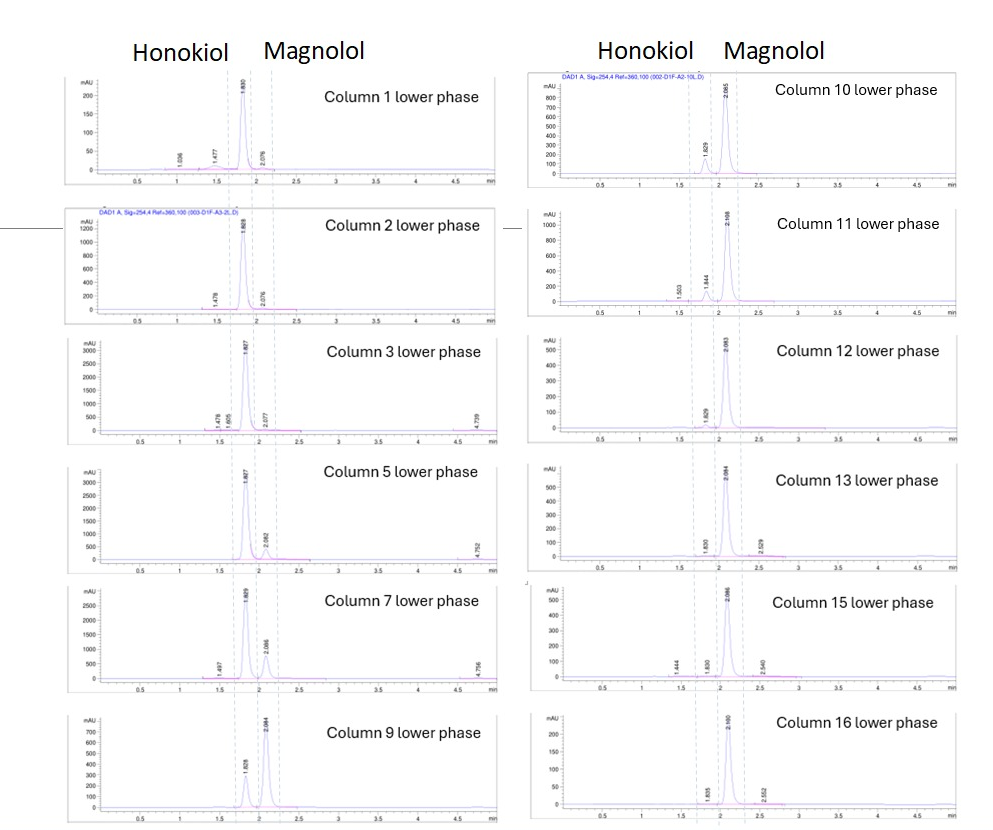
Versatile Across Natural Compounds
Demonstrated broad applicability to natural-origin active compounds, supporting both R&D and pilot-to-industrial transition.
